Abstract
Introduction: RRMM is associated with poor prognosis and decreased overall survival. Chimeric antigen receptor (CAR) T cell therapies have emerged as a potential single-infusion treatment option for these patients. Ide-cel (bb2121), a B-cell maturation antigen (BCMA)-directed CAR T cell therapy for treatment of RRMM, has demonstrated frequent, deep, and durable responses in heavily pretreated patients in the KarMMa clinical trial (Munshi NC, et al. N Engl J Med 2021;384:705-716). However, evidence on long-term healthcare resource utilization (HCRU) and associated costs of care following ide-cel administration is very limited; this abstract provides a long-term update to a previous 12-month (mo) cost-of-care analysis (Hari P, et al. Value Health 2021;24[Suppl 1]:S29-S30). The objective of this study was to estimate the 2-year (y) post-infusion cost of care for patients with RRMM who received ide-cel in the KarMMa trial.
Methods: This retrospective study used the KarMMa clinical trial database to analyze the HCRU and cost associated with ide-cel. We identified resource use and employed a micro-costing methodology to estimate post-infusion costs. Beginning on the index date (day of ide-cel infusion) up to a 2-y follow-up period, we analyzed the following HCRU categories: facility (standard inpatient [IP] and intensive care unit [ICU]), diagnostic (eg, lab work and imaging), medication (eg, supportive care, prophylactic treatment, and adverse event management), and procedure (eg, dialysis and intubation); and length of stay (LOS) (number of standard IP days and ICU days). Given that some patients in the trial were administered ide-cel outside the United States (US), any HCRU inconsistent with US clinical practice were adapted to reflect the US perspective. Next, we applied unit costs to each HCRU in the time period. Unit costs were from the US health system (provider) perspective and adjusted to 2022 USD. Cost per standard IP day ($2944) was estimated from 2018 Healthcare Cost and Utilization Project databases and cost per ICU day ($8426) sourced from Dasta JF, et al. Crit Care Med 2005;33:1266-1271. Medication cost data were obtained from REDBOOKTM using wholesale acquisition cost and were uniformly applied across sites of care. Diagnostic, transfusion, and procedure costs were taken from the Centers for Medicare & Medicaid Services lab fee schedule, physician fee schedule, or outpatient prospective payment system. Unit costs were estimated by applying a payment-to-cost ratio to any Medicare reimbursement rates. Costs were then adjusted to reflect cost to the site of care where the HCRU occurred. Costs were aggregated by HCRU category (facility, medication, procedure, and diagnostic) and partitioned by mo following ide-cel administration. An average total cost by post ide-cel infusion mo was calculated among patients with ongoing status in that mo. Patients censored because of data cut off were excluded.
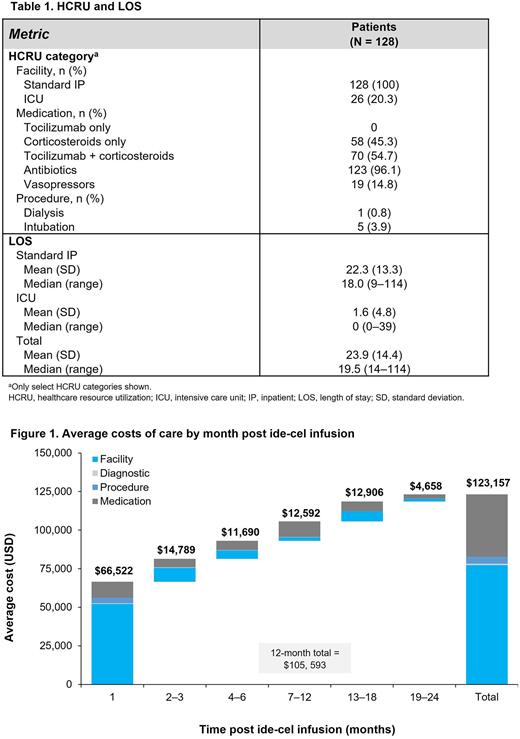
Results: A total of 128 patients were treated with ide-cel in the KarMMa trial. Patients had a mean (standard deviation [SD]) age of 59.8 (9.3) y, and were predominantly male (59.4%), White (80.5%), and non-Hispanic (80.5%). HCRU and LOS, including standard IP and ICU days, are shown in Table 1. Mean (SD) LOS throughout the follow-up period was 22.3 (13.3) days for standard IP and 1.6 (4.8) days for ICU. Total LOS ranged from 14 to 114 days with a median of 19.5 days and a mean (SD) of 23.9 (14.4) days (protocol required IP stay ≥ 14 days post infusion). HCRU, especially in y 2 and for medications, may have been related to management of toxicity or progressive disease. All patients were hospitalized (per trial protocol); 20.3% had an ICU stay. Most patients received antibiotics (96.1%) and tocilizumab + corticosteroids (54.7%), some received corticosteroids only (45.3%), and few had vasopressor use (14.8%). A minority of patients required dialysis (0.8%) or intubation (3.9%). The estimated mean 2-y post-infusion cost of care was $123,157. Most of the 2 y costs post-infusion were incurred in y 1 (85.7%) with 54% in mo 1 and were driven primarily by facility costs, namely standard IP and ICU stays (Figure 1).
Conclusions: This study is the first to estimate long-term (national average-based) cost of care following CAR T cell therapy for patients with RRMM. Findings suggest that a single infusion of ide-cel is associated with substantially reduced HCRU and long-term costs following the initial treatment period.
Disclosures
McGarvey:BMS: Research Funding; Amgen: Research Funding; Abbvie: Research Funding; Iovance: Research Funding; Omeros: Research Funding; Grail: Research Funding. Carattini:BMS: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Sanofi: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Ung:BMS: Current Employment, Current equity holder in publicly-traded company. Campbell:BMS: Current Employment, Current equity holder in publicly-traded company. Patwardhan:BMS: Current Employment; Novartis: Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal